The first time Dr. Peter Hackett saw a patient with frostbite, the man died from his injuries. It was in Chicago in 1971, and the man had gotten drunk and passed out in the snow, his fingers so frozen that gangrene eventually set in.
Dr. Hackett later worked at Everest Basecamp, Denali, Alaska, and now in Colorado, becoming an expert in treating cold injuries. The experience was often the same: There wasn’t much that could be done about frostbite except rewarm the patient, give aspirin, amputate in severe cases, and, more often, wait and accept that six months later the patient’s body can “automatically lean” into naturally dropping a dead finger or toe.
His mentor in Anchorage used to say, “Frost January, Amputation July,” recalled Dr. Hackett, a clinical professor at the Altitude Research Center at the University of Colorado Anschutz Medical Campus. “For centuries, there was nothing else to do.”
This month, the Food and Drug Administration approved the first treatment for severe frostbite in the country. The drug, iloprost, is given intravenously for several hours a day for a little over a week. It works by opening blood vessels to improve circulation, reduce inflammation and stop the formation of platelet clumps that can stop circulation and kill tissue. Most at risk are a person’s toes, fingers, ears, cheeks and nose.
Treatment approval is both scientific innovation and a money-making bonus for the pharmaceutical industry. Experts say there is no good data on how many people have frostbite severe enough to receive this treatment. But the cases could be as many as a few dozen people a year in the United States, according to Dr. Norman Stockbridge, chief of the FDA’s division of cardiology and nephrology at the agency’s Center for Drug Evaluation and Research, which approved the drug.
“When you come across people who are really frozen and really at risk of losing digits, it’s quite unusual,” said Dr. Stockbridge. However, “it’s better to have a medicine for it than nothing.”
In fact, the drug’s approval for frostbite highlights an untold reality of the seriousness of the injury: It’s rare.
Most at risk are high-altitude climbers, people working outdoors without proper equipment, and people who are homeless, especially those with poor traffic. Frostbite occurs in “extremely cold temperatures,” according to the Centers for Disease Control and Prevention, with injury often occurring during the thawing process as blood vessels are damaged by clots and inflammation, throttling blood flow.
About two-thirds of all cases of frostbite are milder, sometimes known as frostbite, and are not likely candidates for this drug, according to Allison Widlitz, the vice president of medical affairs for Eicos Sciences, a startup in San Mateo, California. which received FDA approval to sell the drug. He estimated that the US market for iloprost would be fewer than 1,000 people a year.
“Although a small market, this is an important new option,” he said. Eicos, which has seven employees, has not yet set a price for the drug, Ms. Widlitz said.
Many infusion treatments for such rare conditions are very expensive. Treatment with iloprost would involve IVs for six hours a day and up to eight days.
Ms. Widlitz added that the company was founded to explore iloprost and drugs for other unmet medical needs.
This is not the first use of the drug. An inhaled version of iloprost was first approved in 2004 by the FDA for the treatment of pulmonary hypertension. In the last decade, version IV has been approved for severe frostbite in many European countries, after a French doctor, Dr. Emmanuel Cauchy, demonstrated its effectiveness in the treatment of frostbitten mountaineers.
Last year, a publication in The International Journal of Circumpolar Health, a publication devoted to health issues affecting people living in the Arctic Circle, found similar results in later research. He noted that the use of iloprost “demonstrated a reduction in amputation rates relative to untreated patients.”
For example, a 2018 paper published in Wilderness & Environmental Medicine looked at iloprost treatment in five Himalayan climbers and found that the drug prevented tissue loss in two of them and limited tissue loss in two others. Those case studies found the drug effective when given 48 to 72 hours after the onset of injury, an important wrinkle because climbers often can’t get immediate treatment.
In cases where clots are arrested more immediately, a stroke medication called tissue plasminogen activator, or tPA, can be used to limit clot formation and reduce the risk of amputation. However, this drug, if not given within hours, can lead to serious complications and death. Unlike iloprost, tPA is not FDA-approved for severe frostbite, but doctors have resorted to it off-label.
Dr. Hackett said the universe of people who suffer from severe frostbite includes “climbers, snowboarders who stick out, massage therapists, the military” and other people who work in cold conditions, along with those who are homeless and “people with drug and alcohol problems who are exposed to the cold for long periods’.
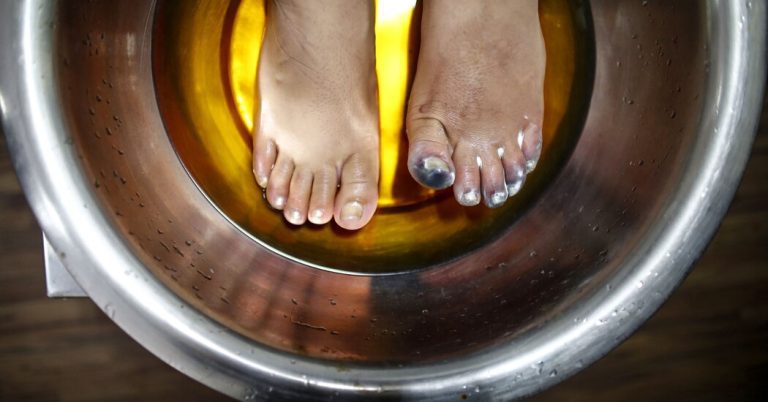
That’s how Boulder, Colo., resident Jennifer Livovich, who was homeless, got severe frostbite on an extremely cold night in December 2016.
He recalled drinking too much and the weather the day before being fine: “Then I woke up the next day, covered in snow, and my shoe had come off while I was sleeping — maybe I cut it — and my left foot was stuck to the ground.”
“I kept walking and I could tell my leg felt different, but I thought I was cold,” he said. Five days later, she ended up in a rehab unit, where, as she warmed up and her leg thawed, “I was in excruciating pain.”
The thawing stage is when damage begins to occur and the capillaries deteriorate, sometimes beyond repair. “Different parts of my leg turned from black to light blue,” he said.
While in the care of a doctor, she tried a warm water soak and elevated her foot, putting gauze between her toes so the regenerating skin cells wouldn’t fuse together. Pieces of skin fell off and she lost all her nails. When the doctors were finally satisfied that the foot had healed as far as it could go, “they shaved — that’s what they call it, ‘shaved off’ — a quarter of an inch off my big toe,” he said.
The shave happened over the summer, roughly matching the six-month timeline according to the saying of Dr.’s mentor. Hackett: injury in early winter and crippled until summer.
So, however small the market for the new drug, Dr. Hackett hopes he could save a few digits.
“It’s great,” he said. “It may change the old adage.”