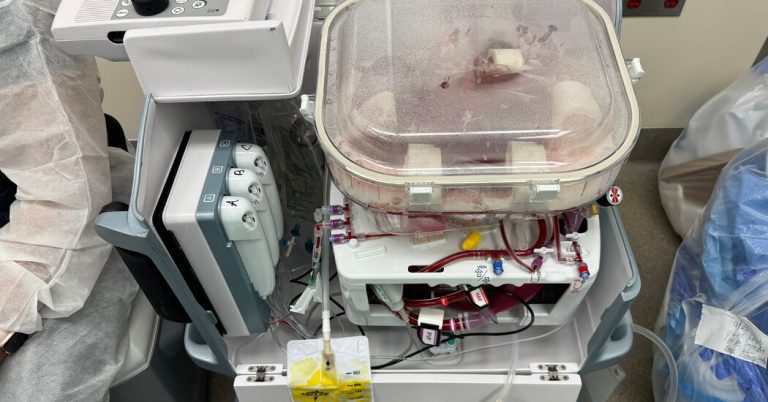
At some level, the human liver in the operating room at Northwestern Memorial Hospital in Chicago was alive. Blood circulating through its tissues supplied oxygen and removed waste products, and the organ produced bile and proteins necessary for the body.
But the donor had died a day earlier and the liver was inside a plastic boxed device. The organ owed its vitality to this machine, which preserved it for transplantation into a needy patient.
“It’s a bit of science fiction,” said Dr. Daniel Borja-Cacho, a transplant surgeon at the hospital.
Surgeons are experimenting with organs from genetically modified animals, hinting at a future in which they could be a source for transplants. But the field is already undergoing a paradigm shift, driven by widely used technologies that allow clinicians to temporarily store organs outside the body.
Perfusion, as it’s called, is changing every aspect of the organ transplant process, from how surgeons operate, to the types of patients who can donate organs, to the outcomes for recipients.
More importantly, surgical programs that have adopted perfusion are transplanting more organs.
As of 2020, Northwestern has seen a 30% increase in liver transplant volume. Nationally, the number of lung, liver and heart transplants increased by more than 10 percent in 2023, one of the largest year-over-year increases in decades.
Without blood flow, organs quickly deteriorate. That’s why clinicians have long considered the ideal organ donor to be someone who died under conditions that stopped brain activity but whose heart continued to beat, keeping the organs viable until they were matched with recipients. .
To minimize injury to organs after they are removed from a donor’s blood supply and before being connected to a recipient, surgeons used to chill them just above freezing, significantly slowing their metabolic processes.
This extends the window in which organs can be transplanted, but only for a while. Livers remain viable for no more than 12 hours, and lungs and hearts closer to six.
Scientists have long experimented with techniques to preserve organs in more dynamic conditions, at a warmer temperature and saturated with blood or other oxygenated solution. After years of development, the first device to preserve lungs by perfusion won approval from the Food and Drug Administration in 2019. Heart and liver perfusion devices were approved in late 2021.
The devices essentially pump blood or an oxygenated fluid through tubes into the blood vessels of the donated organ. Because the cells in a perfused organ continue to function, clinicians can better assess whether the organ will thrive in the recipient’s body.
Bolstered by this information, transplant surgeons began using organs from older or sicker donors that they would otherwise have rejected, said Dr. Kris Croome, professor of surgery at the Mayo Clinic in Florida. “We’re going to look for instruments we’ve never had before, and we’re seeing good results,” he said.
Perfusion also facilitates the grueling process of organ retrieval and transplantation, hours of surgery that doctors often perform around the clock, starting in the middle of the night and completing back-to-back.
Now surgical teams can retrieve an organ, perfuse it overnight while they sleep, and complete the transplant in the morning without fear that the delay will have damaged the organ.
Perhaps most importantly, the transfusion further opened the door to organ donation from comatose patients whose families have withdrawn life support, allowing their hearts to eventually stop. Every year, tens of thousands of people die this way, after the circulation is cut off, but they were rarely potential donors because the dying process deprived their organs of oxygen.
Now, surgeons perfuse these organs, either by removing them in a machine or, in a lower-tech way, by recirculating blood to that area of the donor’s body. And this has made them much more attractive for transplantation.
Since 2020, the number of livers transplanted after the donor’s circulatory death has doubled, according to an analysis of data by the United Network for Organ Sharing, the nonprofit organization that manages the United States’ transplant system.
At one time, surgeons never used hearts from such donors because of the organ’s sensitivity to oxygen deprivation. in 2023, thanks to blood transfusion, they transplanted over 600.
By tapping into this new pool of donors, transplant centers said they could find organs more quickly for surplus patients in urgent need. Dr. Shimul Shah said the organ transplant program he runs at the University of Cincinnati had virtually eliminated his waiting list for livers. “I never thought, in my career, that I would ever say that,” he said.
One barrier to technology adoption can be cost. At the prices demanded by device manufacturers today, injecting an organ outside the body can add more than $65,000 to the price of a transplant. Smaller hospitals may not be able to justify the initial expense.
One of the leading companies, TransMedics, raised its prices significantly after regulators approved its device, prompting a scathing letter from Rep. Paul Gosar, R-Ariz., who wrote: “What started as a promising innovation of medical equipment and an opportunity to increase transplantation nationwide is now being held hostage by a public company that has lost its true north.”
But some surgeons said the technology can still save money, as patients who receive infusion devices generally leave the hospital faster and with fewer complications and have better mid- and long-term outcomes.
Surgeons are still exploring the upper limits of how long hematopoietic organs can survive outside the body, and while the technologies are already fundamentally changing transplantation, some say this is just the beginning.
Dr. Shaf Keshavjee, a surgeon at the University of Toronto whose lab has been at the forefront of developing technologies to keep lungs outside the body, said the devices could eventually allow doctors to remove, repair and return lungs to sick patients rather than replace them. “I think we can make instruments that will outlive the recipient you put them in,” he said.
Dr. Ashish Shah, the chairman of cardiac surgery at Vanderbilt University, one of the nation’s busiest heart transplant programs, agreed, calling it “the holy grail.”
“Your heart is bad,” he said. “I’m taking it out. I put it on my device. While you have no heart, I can support you with an artificial heart for a while. Then I take your heart and fix it – cells, mitochondria, gene therapy, whatever – and then I sew it back in. Your heart. That’s what we’re really working towards.”